Introduction: From Regulatory Chore to Strategic Imperative
In the high-stakes world of medical device, pharmaceutical, and biotech companies, the dual pressures of rapid innovation and stringent regulatory compliance are a constant challenge. Success hinges not only on groundbreaking products but also on the integrity of the processes used to create them. At the heart of this challenge is Title 21 of the Code of Federal Regulations Part 11 or 21 CFR Part 11, a regulation set forth by the U.S. Food and Drug Administration (FDA) that establishes the criteria for electronic records and signatures to be considered trustworthy, reliable, and equivalent to their paper counterparts.
Historically, many organizations have approached compliance as a reactive chore, treating it as a separate, manual, and often painful process. This mindset leads to fragmented data, siloed teams, and an audit preparation process that feels more like "archaeology" than a standard procedure. Quality management becomes a simple "checkbox" exercise, reducing it from a strategic asset to a burdensome obligation.
However, a modern approach to quality management understands that compliance is not an add-on; it is a foundational element of a robust and efficient system. The FDA's original intent behind CFR Part 11 was to mitigate the risk of falsification, misinterpretation, and unauthorized changes that they believed were higher with electronic records than with paper. Consequently, the true measure of a solution is not merely whether it has a list of features, but whether its core design fundamentally addresses these risks.
Unifize enters this arena as a next-generation Electronic Quality Management System (EQMS) that was "engineered to enhance visibility, flexibility, and accountability". By adopting a philosophy of "compliance-by-design, not bolt-on," the Unifize platform transforms CFR Part 11 adherence from a bureaucratic hurdle into a powerful tool for risk mitigation and business acceleration.
For a complete overview of CFR Part 11, explore our Definitive Guide to 21 CFR Part 11.
The Unifize Advantage: A Solution Built for Compliance-by-Design
Traditional EQMS tools often treat compliance as a separate layer of functionality. In contrast, the Unifize platform was built with a fundamental philosophy of "compliance-by-design". This means that the core architecture is inherently structured to produce audit-ready records and ensure regulatory adherence, rather than retrofitting features onto an existing system.
The cornerstone of this approach is a concept referred to as "unified context." Legacy quality management systems typically store documents and records in one place while the critical discussions, approvals, and rationale occur in scattered "side channels" like email, chat, and spreadsheets. This leads to a phenomenon known as "context evaporation," where the "why" behind an approval or a decision is lost, turning audits into a difficult exercise in data archaeology. Unifize addresses this problem by fusing records, chat threads, approvals, and attachments into a single, living "smart object". This eliminates silos and ensures the complete picture—including the full decision history—is instantly available to anyone who needs it and only to those who should.
The platform's unified design also positions collaboration as a central driver of compliance. Unifize is "chat-driven" and builds collaboration into every step of the workflow, from non-conformance reports to change requests. By enabling engineers, suppliers, and auditors to communicate in-line on a process record, it accelerates decision-making and ensures accountability sticks. This integrated, collaborative approach streamlines scattered information and ensures that compliance is a natural byproduct of efficient, connected teamwork.
A Deep Dive into Unifize’s CFR Part 11 Features
1. Scope of CFR Part 11
Unifize's Take: Understanding the breadth of this regulation, Unifize ensures all electronic records, electronic signatures, and even handwritten signatures executed to electronic records meet the integrity, authenticity, and confidentiality standards expected of our paper counterparts.
2. Validation of Software
Unifize's Approach: With an innate system validation mechanism, Unifize assures users of its accuracy, reliability, and consistent performance. The software is designed from the ground up with CFR Part 11 validation criteria in mind.
3. Operational System Checks
Unifize’s Implementation: With embedded operational checks, Unifize guides users through the correct sequencing of processes, ensuring workflow accuracy and compliance.
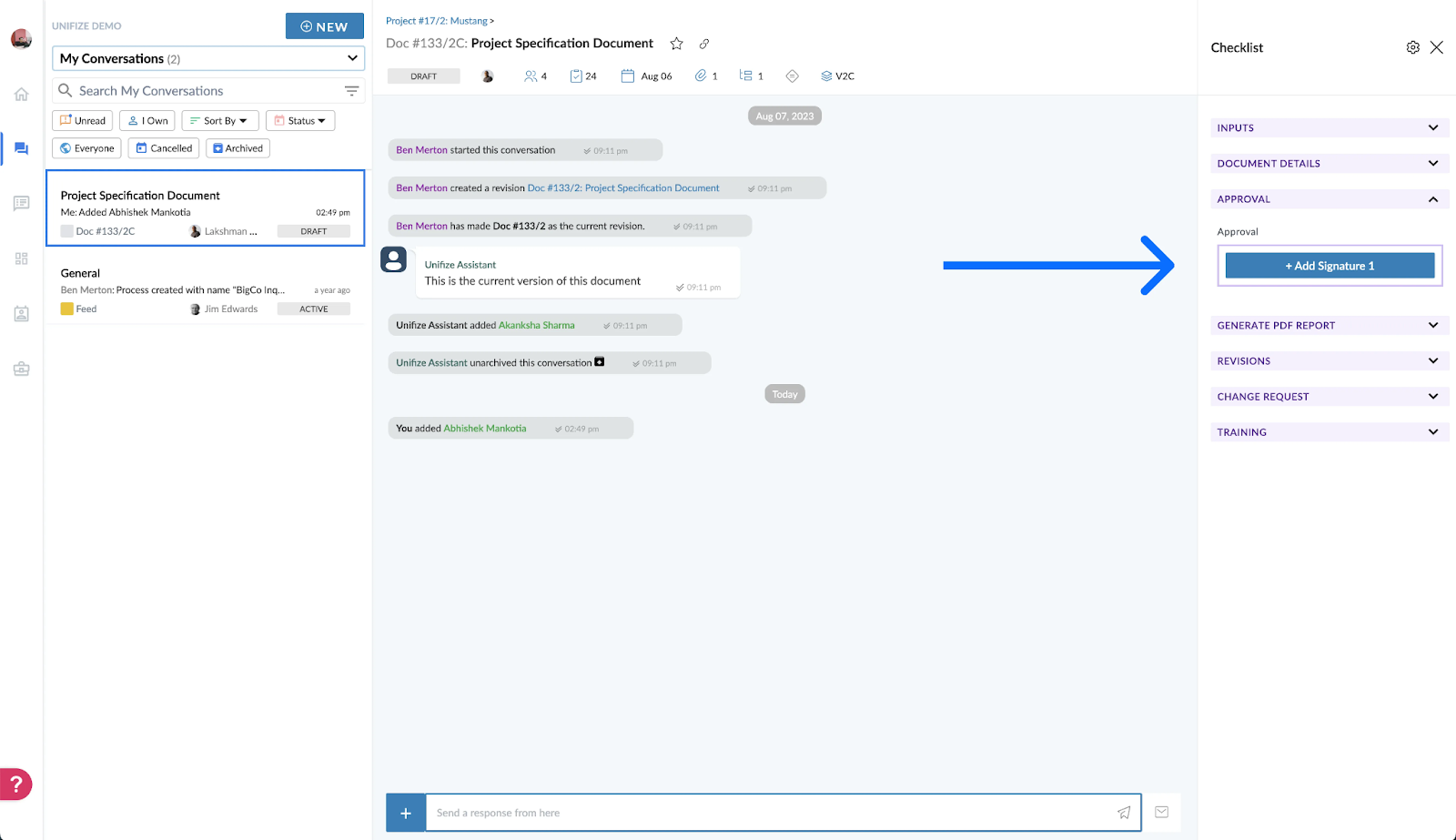
4. Record Retention and Protection
Unifize’s Stance: With Unifize, records aren’t just stored; they are safeguarded. The eQMS ensures prolonged record retention in alignment with regulatory stipulations, bolstered with advanced backup and restoration features to ward off potential data losses.
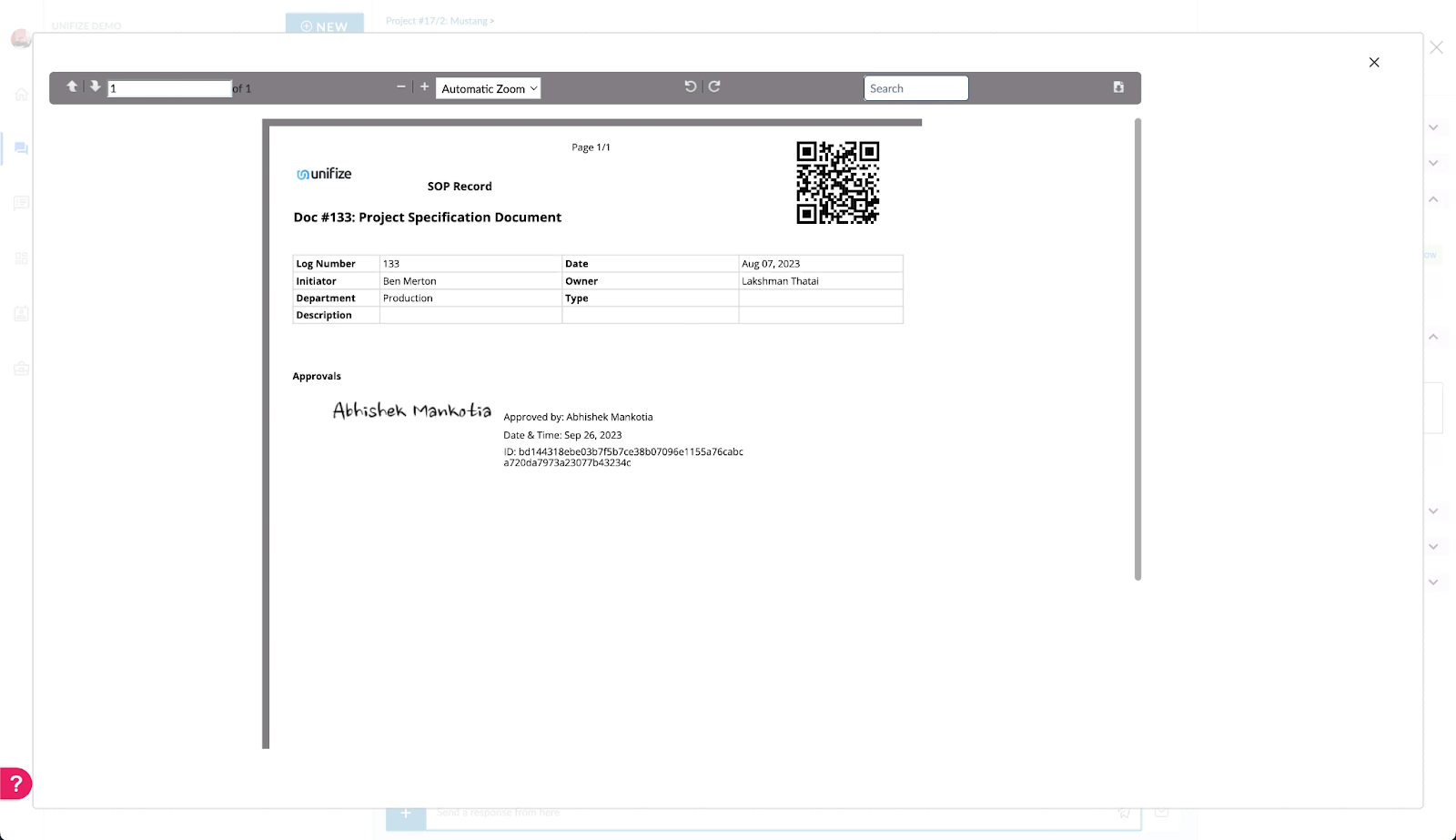
5. Secure Electronic Signatures
Unifize’s Feature: Incorporating top-tier security protocols, Unifize mandates unique user IDs and impenetrable passwords for electronic signatures. Additionally, it introduces measures to verify user identities before any signature execution.

6. System Access Security
Unifize’s Defence Mechanism: Only the authorized get access. Be it through username-password gateways, or two-factor authentications, Unifize prioritizes security, ensuring data sanctity.
7. Audit Trails
Unifize's Solution: Unifize champions transparency by incorporating secure, computer-generated, time-stamped audit trails. Every creation, modification, or deletion of electronic records is tracked meticulously, guaranteeing authenticity at all times.
8. Training for Users
Unifize’s Initiative: Recognizing the complexities of CFR Part 11, Unifize incorporates tailored training modules, ensuring every user is well-versed in the regulation's intricacies and its implications on their roles.
9. Documentation and Procedures
Unifize’s Infrastructure: At Unifize, documentation isn't an afterthought. The platform supports comprehensive procedure creation, review, and approval processes, seamlessly accommodating change controls and system maintenance practices.
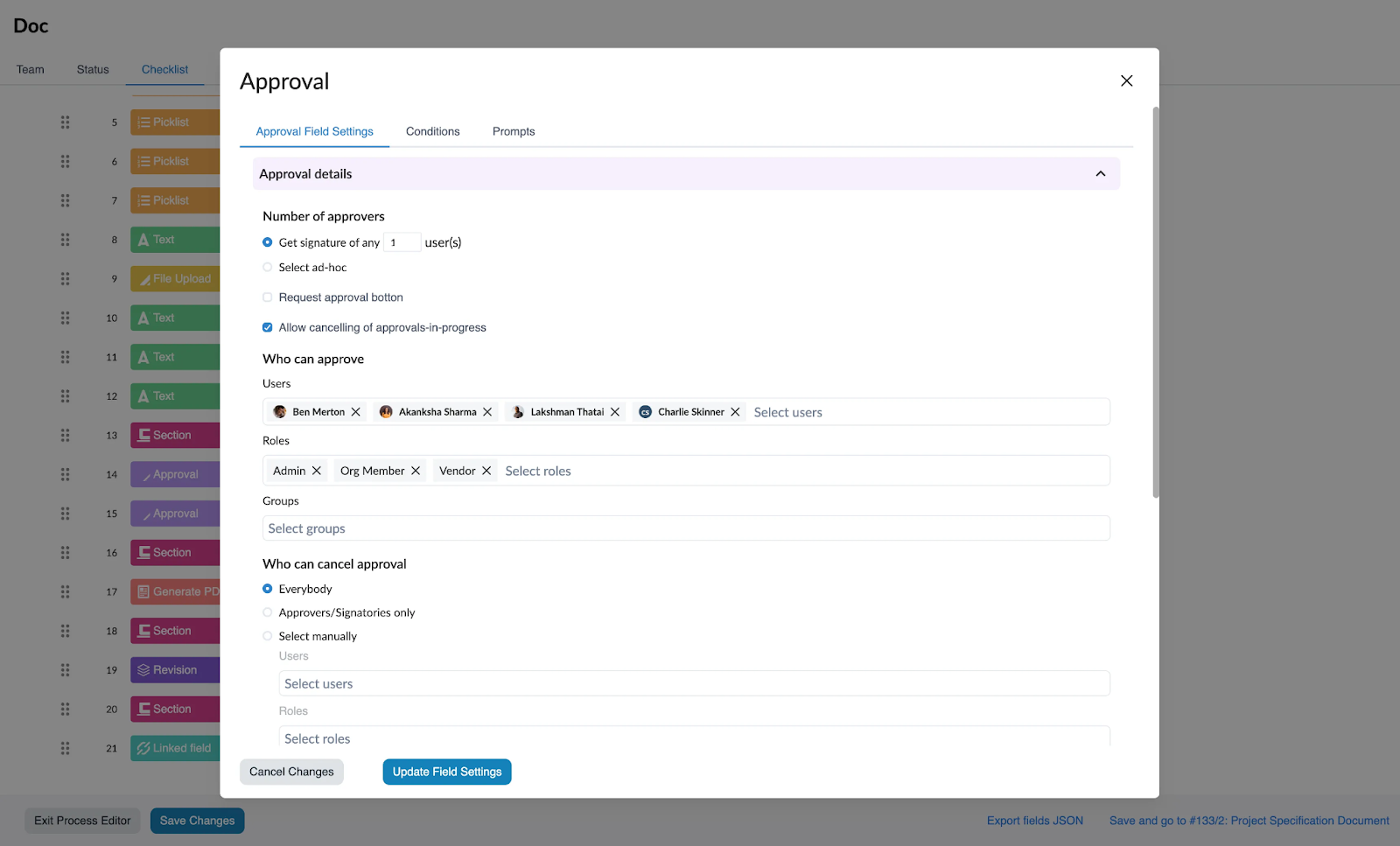
10. Multi-Step Configurable Approval Workflows
Unifize’s Efficiency: Unifize can be configured to any organizational approval workflow while ensuring compliance with CFR Part 11. This facilitates seamless collaboration for efficient review, notification, routing and approval of documents, to the right individual, teams or roles across multiple levels of approvers. This enhances operational efficiency by cutting out unnecessary approval steps and emails, while also maintaining compliance to CFR Part 11.
11. Vendor Support
Unifize’s Promise: Unifize is not just a tool; it's a partnership. Our dedicated support team possesses a deep understanding of regulatory nuances, ensuring that clients navigate the CFR Part 11 landscape confidently and compliantly.
12. Flexibility and Scalability
Unifize’s Vision: Unifize looks to the future. Built to adapt, it welcomes changes in regulatory requirements, ensuring that companies can scale their operations without regulatory hiccups.
13. Integration with Other Systems
Unifize’s Compatibility: In the digital age, integration is king. Unifize understands this, allowing for seamless integrations with other vital operational software without compromising any aspect of CFR Part 11.
Conclusion: Compliance as a Competitive Edge
In a world where digital systems are the new standard, CFR Part 11 compliance is not optional. However, the choice of an EQMS determines whether compliance will be a reactive burden or a strategic advantage. Unifize is a modern, collaborative platform that meets every facet of CFR Part 11 by design, ensuring the integrity and reliability of electronic records and signatures.
By fusing data and collaboration, Unifize creates a unified, audit-ready chronology that eliminates silos and enables actionable analytics. The platform's user-friendly design and proven ability to accelerate processes and reduce costs solidify its position as a strategic asset. In a rapidly evolving industry, the right EQMS can be the difference between an organization that is merely compliant and one that is a proactive, data-driven leader. Unifize enables this transformation, turning compliance from a dreaded chore into a source of innovation, visibility, and accountability.




